Over the years, there have been countless papers written on the importance of glutathione (GSH) and the myriad of ways to supplement this free radical scavenger. By now, we are all aware of its significance in keeping us healthy, but, unfortunately, there are many myths on how to enhance cellular glutathione (GSH) effectively.
We have already discounted the most apparent strategy in one of our articles in which we discuss why taking glutathione itself will not increase its concentration inside the cell. So, let’s move on to the most often quoted myth that the amino acid cysteine is in limited supply in the body. We are aware that cysteine is one of the three building blocks that make up glutathione, but is there any evidence to suggest that we may be low on cysteine? And, regardless, would taking cysteine be effective in increasing cellular glutathione (GSH)? On initial observation, the principle behind the theory of cysteine deficiency being a cause of low glutathione (GSH) appears reasonably sound, but it is not that simple.
The first question is relatively easy to answer. The fact is that our diet usually contains plenty of cysteine and the other sulphur containing amino acid called methionine which can be easily converted into cysteine in the liver [1]. For example, the typical American diet supplies much more than the recommended required quantity of cysteine [2]. We can, therefore, rule out a cysteine deficiency. But would taking a cysteine supplement such as N-acetylcysteine (NAC) increase our cellular glutathione (GSH)? Unfortunately, it is not that easy, otherwise none of the chronic diseases attributed to low glutathione (GSH) would be so prevalent.
Cysteine, unlike most other amino acids, is extremely unstable and rapidly autoxidizes to cystine which is the oxidized disulphide form. It has exceedingly low solubility, and it will not be absorbed Cysteine, unlike most other amino acids, is extremely unstable and rapidly autoxidizes to cystine which is the oxidized disulphide form of cysteine. Cystine has exceedingly low solubility, and it will not be absorbed from the GI tract. Additionally, this cysteine autoxidation reaction, catalyzed by transition metal ions, generates oxygen free radicals and hydrogen peroxide. In high concentrations, this may result in cellular toxicity [3-6] and has the potential to be neurotoxic [7]. Our cells have adapted to this potential toxicity by storing cysteine in the form of glutathione (GSH) [8], which is far more stable to oxidation. We can therefore consider glutathione to be a safe storage for cysteine. It is important to note that consuming cysteine as part of our usual diet will never exceed the threshold to become toxic.
In summary, taking a cysteine supplement is of little use to increase glutathione (GSH) because our body tightly regulates both the storage and production of cysteine and any excess consumed is broken down into more stable byproducts. There is a notable exception which relates to acute glutathione (GSH) depletion due to acetaminophen (paracetamol) overdose as we shall see.
By far the most studied cysteine supplement is the cysteine prodrug N-Acetylcysteine (NAC). Several human clinical studies have determined the bioavailability of NAC. Orally delivered NAC undergoes extensive first-pass metabolism resulting in about 90% loss by enzymatic deacetylation to form cysteine in the small intestine [9]. As we have seen, this mainly gets converted to cystine and is of little use in healthy individuals or those suffering from a chronic undersupply of glutathione (GSH) due to aging or disease. Our notable exception is the observation in several studies that NAC is highly effective in elevating glutathione (GSH) under conditions where there has been a dramatic (acute depletion) drop in intracellular glutathione (GSH) levels, for instance as is the case in acetaminophen overdose. Here, the sharp decline in glutathione (GSH) levels, especially in the liver, to almost zero is effectively counteracted by NAC [10]. It immediately supplies available cysteine for repletion of glutathione and thus, recovery from toxicity. Unfortunately, this is where NAC has gained a false reputation as a go-to drug if low glutathione (GSH) is suspected. While immensely helpful indeed, it does not address our problem of supplementing glutathione (GSH) in cases of gradual depletion such as chronic illness or just simply getting older.
In contrast, diseases in which there is a prolonged and chronic decrease in glutathione (GSH) do not respond well to NAC treatment. An example of this is the situation that occurs in HIV/AIDS patients who experience a persistent drop in tissue glutathione (GSH) levels. In a clinical trial of AIDS patients were treated with 1.8 g/day of NAC for two weeks with the glutathione (GSH) status monitored in plasma and lymphocytes. During the treatment, no significant increase in glutathione (GSH) was observed [11]. Similar disappointing observations of HIV patients supplemented with NAC were also made by [12] and [13]. NAC has been tried in numerous chronic diseases with similarly disappointing results including cystic fibrosis protection against contrast-induced nephropathy and thrombosis [14].
The tight negative feedback control that glutathione (GSH) exerts on the first of two enzymes responsible for glutathione synthesis, GCL, can explain this phenomenon. This enzyme has the task of combining the amino acids glutamate and cysteine to form gamma-glutamylcysteine (GGC), which is used to produce glutathione (GSH) by the GS enzyme. As long as cellular glutathione is above a level considered to be adequate, which is called homeostasis, GCL is inhibited from making gamma-glutamylcysteine (GGC), no matter how much cysteine is available. However, when intracellular glutathione (GSH) is well below this homeostatic level, GCL is no longer inhibited and can actively utilize the cysteine supplied by NAC supplements. Several researchers have come to the same conclusion when trying to explain this fact [15]. Negative feedback controls exist as part of many of our body’s processes to tightly regulate certain functions, for example, our body temperature.
Supplementing with cysteine to increase cellular glutathione (GSH) is therefore of little use except in a few severe and limited cases mainly used in clinical settings. The causes of glutathione (GSH) depletion in chronic diseases and how to effectively, rapidly and safely augment cellular glutathione (GSH) is now well understood.
References
- Courtney-Martin, G., R.O. Ball, and P.B. Pencharz, Sulfur amino acid metabolism and requirements. Nutrition Reviews, 2012. 70(3): p. 170-175.
- Lang, C.A., The impact of glutathione on health and longevity. Journal of Anti Aging Medicine, 2001. 4(2): p. 137-144.
- Nath, K.A. and A.K. Salahudeen, Autoxidation of cysteine generates hydrogen peroxide: cytotoxicity and attenuation by pyruvate. American Journal of Physiology, 1993. 264(2): p. F306-F314.
- Harman, L.S., C. Mottley, and R.P. Mason, Free radical metabolites of L-cysteine oxidation. Journal of Biological Chemistry, 1984. 259(9): p. 5606-11.
- Viña, J., et al., The effect of cysteine oxidation on isolated hepatocytes. Biochem. J., 1983. 212(1): p. 39-44.
- Wang, X.F. and M.S. Cynader, Pyruvate Released by Astrocytes Protects Neurons from Copper-Catalyzed Cysteine Neurotoxicity. The Journal of Neuroscience, 2001. 21(10): p. 3322-3331.
- Janáky, R., et al., Mechanisms of L-Cysteine Neurotoxicity. Neurochemical Research, 2000. 25(9): p. 1397-1405.
- Aoyama, K., M. Watabe, and T. Nakaki, Regulation of Neuronal Glutathione Synthesis. Journal of Pharmacological Sciences, 2008. 108(3): p. 227-238.
- Olsson, B., et al., Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. European Journal of Clinical Pharmacology, 1988. 34(1): p. 77-82.
- Yang, R.K., et al., Prolonged treatment with N-acetylcystine delays liver recovery from acetaminophen hepatotoxicity. Critical Care, 2009. 13(2).
- Witschi, A., et al., Supplementation of N-acetylcysteine fails to increase glutathione in lymphocytes and plasma of patients with AIDS. AIDS Research & Human Retroviruses, 1995. 11(1): p. 141-3.
- Akerlund, B., et al., Effect of N-acetylcysteine(NAC) treatment on HIV-1 infection: a double-blind placebo-controlled trial. European Journal of Clinical Pharmacology., 1996. 50(6): p. 457-61.
- Nakamura, H., H. Masutani, and J. Yodoi, Redox imbalance and its control in HIV infection. Antioxidants & Redox Signaling, 2002. 4(3): p. 455-64.
- Rushworth, G.F. and I.L. Megson, Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacology & Therapeutics, 2014. 141(2): p. 150-159.
- Nielsen, H.B., et al., N-acetylcysteine does not affect the lymphocyte proliferation and natural killer cell activity responses to exercise. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology, 1998. 275(4): p. R1227-R1231.

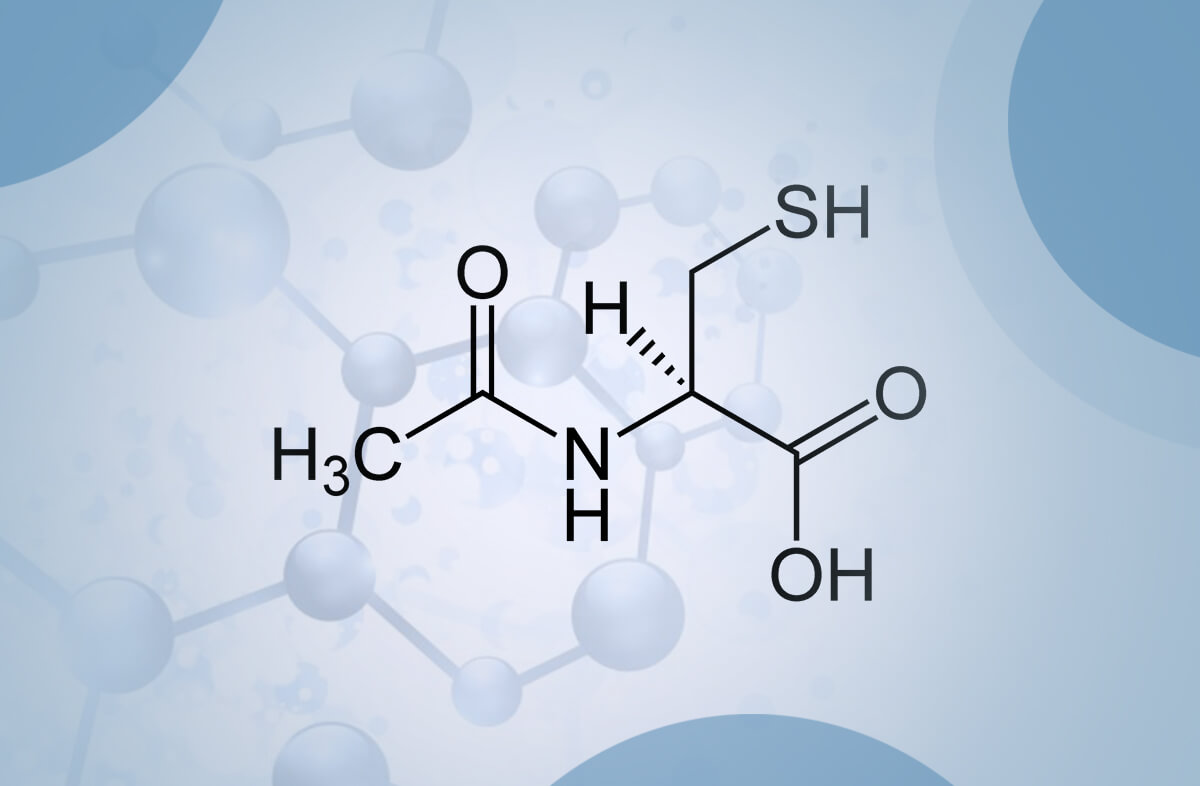
Can you comment on the study done on NAC Ethyl Ester? The esterification of the carboxyl group of NAC leads to a significantly higher lipophilicity and bioavailability, allowing this compound to increase Glutathione intracellularly. One of the studies below show’s it increases GSH by 300% intracellularly.
I’d love to see an article on this novel compound.
https://www.sciencedirect.com/science/article/abs/pii/S0006295212006417?via%3Dihub
https://www.sciencedirect.com/science/article/abs/pii/S0891584918311055?via%3Dihub
Hi Matt,
Firstly, you need to take into consideration that both NAC and NAC ethyl ester are drugs – they are not natural – NAC is commonly marketed as a dietary supplement, but this is the subject of a current investigation by the USA’s FDA. It should also be noted that, unlike NAC, NAC ethyl ester is still an experimental drug, with the few published studies limited to cell culture and animal models. No animal toxicity or human bioavailability studies have as yet been reported in the literature. Why it is available for sale as a dietary supplement is a regulatory mystery as it does not seem to have FDA approval or GRAS status.
NAC was first introduced as a drug in the 1960s for the treatment of acetaminophen/paracetamol overdose, which acutely depletes glutathione in the liver. Cysteine is the rate-limiting amino acid in glutathione synthesis (i.e., the first one to run out) but cysteine itself can be toxic, particularly in the high doses required for treating such overdoses. NAC is considered a less toxic form and hence a safer treatment. The papers you cite provide evidence that NAC ethyl ester is more bioavailable than NAC i.e., it is capable of delivering more cysteine to cells than NAC and the authors suggest that this may make NAC ethyl ester a superior antidote, which they have explored in another report. The problem that NAC ethyl ester will have in replacing NAC as the approved antidote is gaining ethics approval for the required human clinical studies. Given that NAC is effective in treating overdoses, attempting to gain informed consent, from those suffering an overdose, to try something different with unknown risks and without clinical evidence of efficacy in a clinical trial should not be considered ethical. Most of the authors’ findings are based on NAC ethyl ester being more bioavailable than NAC to help cells that have been acutely depleted of glutathione (e.g., through drug overdose) get back to homeostasis. However, for those wishing to increase their glutathione levels above homeostasis then the only theoretical and clinically (human) demonstrated option is gamma-glutamylcysteine, which is the immediate natural precursor to glutathione and provides a unique option for bypassing the regulatory control of cellular glutathione homeostasis. Unlike NAC and NAC ethyl ester, gamma glutamylcysteine is ubiquitous in nature – it is produced by every cell of all life that uses oxidative phosphorylation (oxygen) to release energy. It is also a significant component of mammalian milk and egg whites and as such is an inarguable food component. We will most certainly look to including an article that describes the research, opportunities, and limitations of NAC ethyl ester
why not just take glutathione directly, it has come a long way lately and very stable in this nano form, bypassing the gut etc, do more research, neumi.com/dawnnz
Dear Dwan, From what I understand of the product you are talking about is so-called nano-sized glutathione in a water solution that you swish in your mouth. Unfortunately, the producer of this product has not understood that it is impossible to get nano-sized particles of glutathione in water suspension because glutathione itself is a very water-soluble compound so as soon as those crystals of glutathione, whether they are nano-sized or normal-sized touch the water they dissolve rapidly. All they are selling is a solution of glutathione in water with a few other ingredients and flavoring thrown in. This is no different from taking a solid supplement of glutathione that dissolves in your stomach anyway. The details of why glutathione supplementation cannot increase cellular glutathione levels can be seen at this article:- https://www.glutathionereporter.com/glutathione-supplementation-cannot-increase-cellular-glutathione-levels/
Please comment on this recent study showing NAC and glycine taken in combination do raise GSH. Seems promising.
Glycine and N‐acetylcysteine (GlyNAC) supplementation in older adults improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, insulin resistance, endothelial dysfunction, genotoxicity, muscle strength, and cognition: Results of a pilot clinical trial
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8002905/ Clinical and Translational Medicine
There is no scientific or biochemical reason to mix NAC and glycine since cells always contain more than enough glycine to form glutathione. In fact, both cysteine and glycine are not only non-essential amino acids that our body synthesises, but they are also abundant in our diets. There have been countless unsuccessful attempts to increase cellular glutathione with NAC and several hundred published papers confirm this finding. Adding glycine is just another marketing hype that ignores the fact that, nutritionally, a lack of glycine is not the reason why glutathione may be low.
The research of this mixture has many inaccuracies and the data presented does not lead to the conclusion that NAC + glycine will increase cellular glutathione levels. More importantly, none of the amino acids that make up glutathione are really ever in undersupply. Glutathione levels can become depleted because the first step enzyme in glutathione synthesis that makes gamma-glutamylcysteine is dysfunctional. Throwing more amino acids into the mix will not make a difference
Thank you for cutting through the hype. Should I assume in the future that the journal of Clinical and Translational Medicine is not a reliable peer reviewed publication? I also missed the connection that the study site, Baylor School of Medicine, held the patent for GlyNAC. Distressing that this medical school would lend its name to this flawed study.
It’s certainly disappointing to see that both the reviewers and editors of the Journal of Clinical and Translational Medicine and the Baylor School of Medicine allow such publications to be made. It does point to one observation that researchers are desperate to find some way to increase cellular glutathione. It’s just a shame that their quest is often misguided and clouded by commercial imperatives.